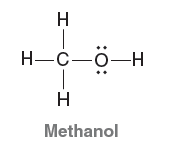
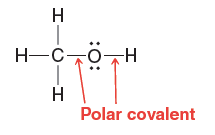
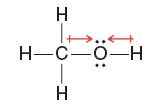
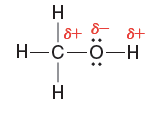
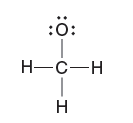
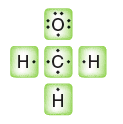
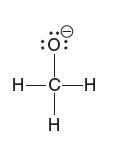
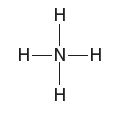
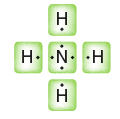
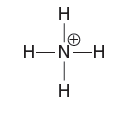
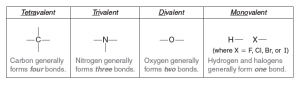
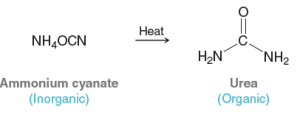
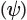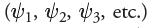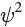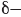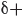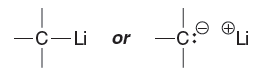Quantum Mechanics
By the 1920s, vitalism had been discarded. Chemists were aware of constitutional isomerism and had developed the structural theory of matter. The electron had been discovered and identified as the source of bonding, and Lewis structures were used to keep track of shared and unshared electrons. But the understanding of electrons was about to change dramatically.
In 1924, French physicist Louis de Broglie suggested that electrons, heretofore considered as particles, also exhibited wavelike properties. Based on this assertion, a new theory of matter was born. In 1926, Erwin Schrödinger, Werner Heisenberg, and Paul Dirac independently proposed a mathematical description of the electron that incorporated its wavelike properties. This new theory, called wave mechanics, or quantum mechanics , radically changed the way we viewed the nature of matter and laid the foundation for our current understanding of electrons and bonds.
Quantum mechanics is deeply rooted in mathematics and represents an entire subject by itself. The mathematics involved is beyond the scope of our course, and we will not discuss it here. However, in order to understand the nature of electrons, it is critical to understand a few simple highlights from quantum mechanics:
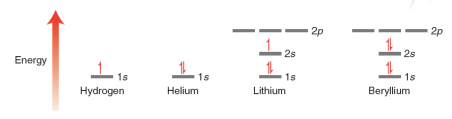
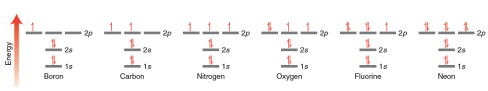
— An equation is constructed to describe the total energy of a hydrogen atom (i.e. proton plus one electron). This equation, called the wave equation, takes into account the wavelike behavior of an electron that is in the electric field of a proton.
— The wave equation is then solved to give a series of solutions called wave function . The Greek symbol psi 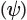 is used to denote each wave function
is used to denote each wave function 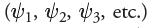 . Each of these wavefunctions corresponds to an allowed energy level for the electron. This result is incredibly important because it suggests that an electron, when contained in an atom, can only exist at discrete energy levels
. Each of these wavefunctions corresponds to an allowed energy level for the electron. This result is incredibly important because it suggests that an electron, when contained in an atom, can only exist at discrete energy levels 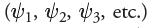 . In other words, the energy of the electron is quantized.
. In other words, the energy of the electron is quantized.
— Each wave function is a function of spatial location. It provides information that allows us to assign a numerical value for each location in three-dimensional space relative to the nucleus. The square of that value (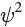 for any particular location) has a special meaning. It indicates the probability of finding the electron in that location. Therefore, a three-dimensional plot of
for any particular location) has a special meaning. It indicates the probability of finding the electron in that location. Therefore, a three-dimensional plot of 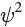 will generate an image of an atomic orbital.
will generate an image of an atomic orbital.
Electron Density and Atomic Orbitals
An orbital is a region of space that can be occupied by an electron. But care must be taken when trying to visualize this. There is a statement from the previous section that must be clarified because it is potentially misleading: “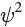 represents the probability of finding an electron in a particular location .” This statement seems to treat an electron as if it were a particle flying around within a specific region of space. But remember that an electron is not purely a particle—it has wavelike properties as well. Therefore, we must construct a mental image that captures both of these properties. That is not easy to do, but the following analogy might help. We will treat an occupied orbital as if it is a cloud—similar to a cloud in the sky. No analogy is perfect, and there are certainly features of clouds that are very different from orbitals. However, focusing on some of these differences between electron clouds (occupied orbitals) and real clouds makes it possible to construct a better mental model of an electron in an orbital:
represents the probability of finding an electron in a particular location .” This statement seems to treat an electron as if it were a particle flying around within a specific region of space. But remember that an electron is not purely a particle—it has wavelike properties as well. Therefore, we must construct a mental image that captures both of these properties. That is not easy to do, but the following analogy might help. We will treat an occupied orbital as if it is a cloud—similar to a cloud in the sky. No analogy is perfect, and there are certainly features of clouds that are very different from orbitals. However, focusing on some of these differences between electron clouds (occupied orbitals) and real clouds makes it possible to construct a better mental model of an electron in an orbital:
— Clouds in the sky can come in any shape or size. However, electron clouds only come in a small number of shapes and sizes (as defined by the orbitals).
— A cloud in the sky is comprised of billions of individual water molecules. An electron cloud is not comprised of billions of particles. We must think of an electron cloud as a single entity, even though it can be thicker in some places and thinner in other places. This concept is critical and will be used extensively throughout the course in explaining reactions.
— A cloud in the sky has edges, and it is possible to define a region of space that contains 100% of the cloud. In contrast, an electron cloud does not have defined edges. We frequently use the term electron density, which is associated with the probability of finding an electron in a particular region of space. The “shape” of an orbital refers to a region of space that contains 90-95% of the electron density. Beyond this region, the remaining 5-10% of the electron density tapers off but never ends. In fact, if we want to consider the region of space that contains 100% of the electron density, we must consider the entire universe.
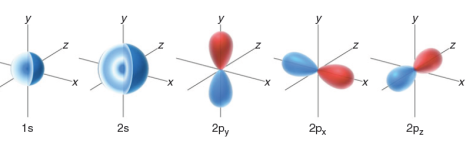
In summary, we must think of an orbital as a region of space that can be occupied by electron density. An occupied orbital must be treated as a cloud of electron density . This region of space is called an atomic orbital (AO), because it is a region of space defined with respect to the nucleus of a single atom. Examples of atomic orbitals are the s , p , d , and f orbitals that were discussed in your general chemistry textbook.
Phases of Atomic Orbitals
Our discussion of electrons and orbitals has been based on the premise that electrons have wavelike properties. As a result, it will be necessary to explore some of the characteristics of simple waves in order to understand some of the characteristics of orbitals.
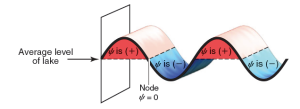
Consider a wave that moves across the surface of a lake. The wave function 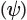 mathematically describes the wave, and the value of the wave function is dependent on location. Locations above the average level of the lake have a positive value for
mathematically describes the wave, and the value of the wave function is dependent on location. Locations above the average level of the lake have a positive value for 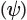 (indicated in red), and locations below the average level of the lake have a negative value for
(indicated in red), and locations below the average level of the lake have a negative value for 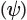 (indicated in blue). Locations where the value of
(indicated in blue). Locations where the value of 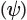 is zero are called nodes.
is zero are called nodes.
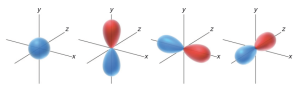
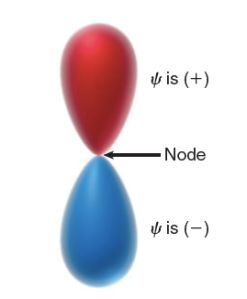
Similarly, orbitals can have regions where the value of 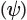 is positive, negative, or zero. For example, consider a p orbital. Notice that the p orbital has two lobes: the top lobe is a region of space where the values of
is positive, negative, or zero. For example, consider a p orbital. Notice that the p orbital has two lobes: the top lobe is a region of space where the values of 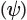 are positive, while the bottom lobe is a region where the values of
are positive, while the bottom lobe is a region where the values of 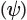 are negative. Between the two lobes is a location where
are negative. Between the two lobes is a location where 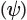 = 0. This location represents a node.
= 0. This location represents a node.
Be careful not to confuse the sign of 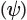 ( + or – ) with electrical charge. A positive value for
( + or – ) with electrical charge. A positive value for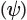 does not imply a positive charge. The value of
does not imply a positive charge. The value of 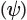 (+or -) is a mathematical convention that refers to the phase of the wave (just like in the lake). Although
(+or -) is a mathematical convention that refers to the phase of the wave (just like in the lake). Although 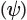 can have positive or negative values, nevertheless
can have positive or negative values, nevertheless 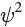 (which describes the electron density as a function of location) will always be a positive number. At a node, where
(which describes the electron density as a function of location) will always be a positive number. At a node, where 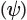 = 0, the electron density
= 0, the electron density 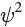 will also be zero. This means that there is no electron density located at a node. From this point forward, we will draw the lobes of an orbital with colors (red and blue) to indicate the phase of
will also be zero. This means that there is no electron density located at a node. From this point forward, we will draw the lobes of an orbital with colors (red and blue) to indicate the phase of 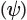 for each region of space.
for each region of space.
 or
or 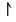 ). In order for the orbital to accommodate two electrons, the electrons must have opposite spin states.
). In order for the orbital to accommodate two electrons, the electrons must have opposite spin states.